Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes
N Engl J Med 2019; 381:1707-1717
October 31, 2019
Sue A Brown, Boris P Kovatchev, Dan Raghinaru, John W Lum, Bruce A Buckingham, Yogish C Kudva, Lori M Laffel, Carol J Levy, Jordan E Pinsker, R Paul Wadwa, Eyal Dassau, Francis J Doyle 3rd, Stacey M Anderson, Mei Mei Church, Vikash Dadlani, Laya Ekhlaspour, Gregory P Forlenza, Elvira Isganaitis, David W Lam, Craig Kollman, Roy W Beck, iDCL Trial Research Group
PMID: 31618560
DOI: 10.1056/NEJMoa1907863
ClinicalTrials.gov: NCT03563313
Introduction
Diabetes mellitus belongs to a group of metabolic disorders characterized by hyperglycaemia. Diabetes mellitus type 1 develops as a result of autoimmune reaction resulting in incomplete or almost total insulin deficiency. DM is the leading cause of the end-stage renal disease (ESRD), nontraumatic lower extremity amputations and adult blindness. It also predisposes to cardiovascular diseases.
As stated in NICE Guidance, aside from hyperglycaemia (>11mmol/l) some of the common symptoms of type 1 diabetes are polydipsia, polyuria, weight loss and extreme tiredness.
Since 2004 there have been major changes to the routine management of type 1 diabetes, in an attempt to achieve much stricter targets for blood glucose control to further reduce the long-term risks associated with the condition. NICE Guidelines recommend attempting to reach a glycated haemoglobin (HbA1c) level near the normal range and near normoglycaemia. This tight control may be achieved by intensive insulin management (multiple daily injections of insulin or insulin pump therapy) from diagnosis, accompanied by carbohydrate counting.
As Brown et al. stated in their study, despite advances in care, attaining good glycemic outcomes in patients with type 1 diabetes remains challenging. The targets set by the American Diabetes Association are met in only a minority of patients (reasonable HbA1C goal for nonpregnant adults is <7%).
This report reviews the effectiveness and safety of a new closed-loop system (also referred to as an “artificial pancreas”), in comparison to the already exerted sensor-augmented pump.
This closed-loop system uses an algorithm with a dedicated hypoglycemia safety module, automated correction boluses, and overnight intensification of basal insulin delivery designed to consistently target near-normal glycaemia each morning.
Methods
Study design
The International Diabetes Closed Loop (iDCL) Trial is a parallel-group, unblinded, randomized trial with 2:1 randomization conducted at seven university centres in the United States.
Funding was provided by the National Institute of Diabetes and Digestive and Kidney Diseases. Tandem Diabetes Care provided the experimental closed-loop systems, supplies, and technical expertise with device issues.
The main objective of the study was to assess the efficacy and safety of the closed-loop system in comparison the sensor-augmented pump in managing the blood glucose level.
Did the trial address a clearly focused issue?
The primary outcome was the percentage of time that the glucose level, as measured by the continuous glucose monitor, was in the target range of 3.9 to 10.0 mmol/l.
Eligibility
To be included in the trial, patients had to meet these criteria:
- be at least 14 years old and have a clinical diagnosis of type 1 diabetes
- they had to have been treated with insulin for at least 1 year using insulin pump or multiple daily injections
- total use of insulin (TDD) had to be at least 10 IU/day
Exclusion criteria:
- if the patient was currently using any non-insulin glucose-lowering agent other than metformin
- haemophilia or any other condition, which would put the patients or the study at risk
- participation in other trials
Deviation from eligibility
At the conclusion of the study, it was identified that one patient in the closed-loop control (CLC) group had been using acarbose, an excluded medication.
Study protocol
After the eligibility of the patients was assessed a total of 168 patients were randomly assigned in a 2:1 ratio to use a closed-loop system (closed-loop group) or a sensor-augmented pump (control group). The trial started with 2-to-8-week run-in phase (it could be skipped by patients familiar with continuous glucose monitoring and insulin pump) and followed by 26-weeks long period of data collecting.
Was the assignment of patients to treatments randomised?
A total of 168 patients underwent 2:1 randomization; 112 were assigned to the closed-loop group, and 56 were assigned to the control group. All 168 patients completed the trial.
Patients in both groups attended in total 4 follow-up visits during 6 months and were contacted 5 times by telephone during the trial period. Data from the devices were downloaded and reviewed at each visit and during telephone contacts. Glycated haemoglobin was measured at each trial site in total of 4 times.
Reporting of adverse events (such as severe hypoglycaemia, diabetic ketoacidosis or hyperglycaemia with ketonemia) were solicited throughout the trial.
Were patients, health workers and study personnel ‘blind’ to treatment?
This trial was not blinded.
Were the groups similar at the start of the trial?
The closed-loop and control groups appeared balanced with regard to baseline characteristics.
After these 26 weeks, followed 3 month extension phase in which the original control group used the closed loop system and the original intervention group was randomly assigned 1:1 to continue closed loop or switch to sensor-augmented pump.
Aside from the experimental intervention, were the groups treated equally?
Yes.
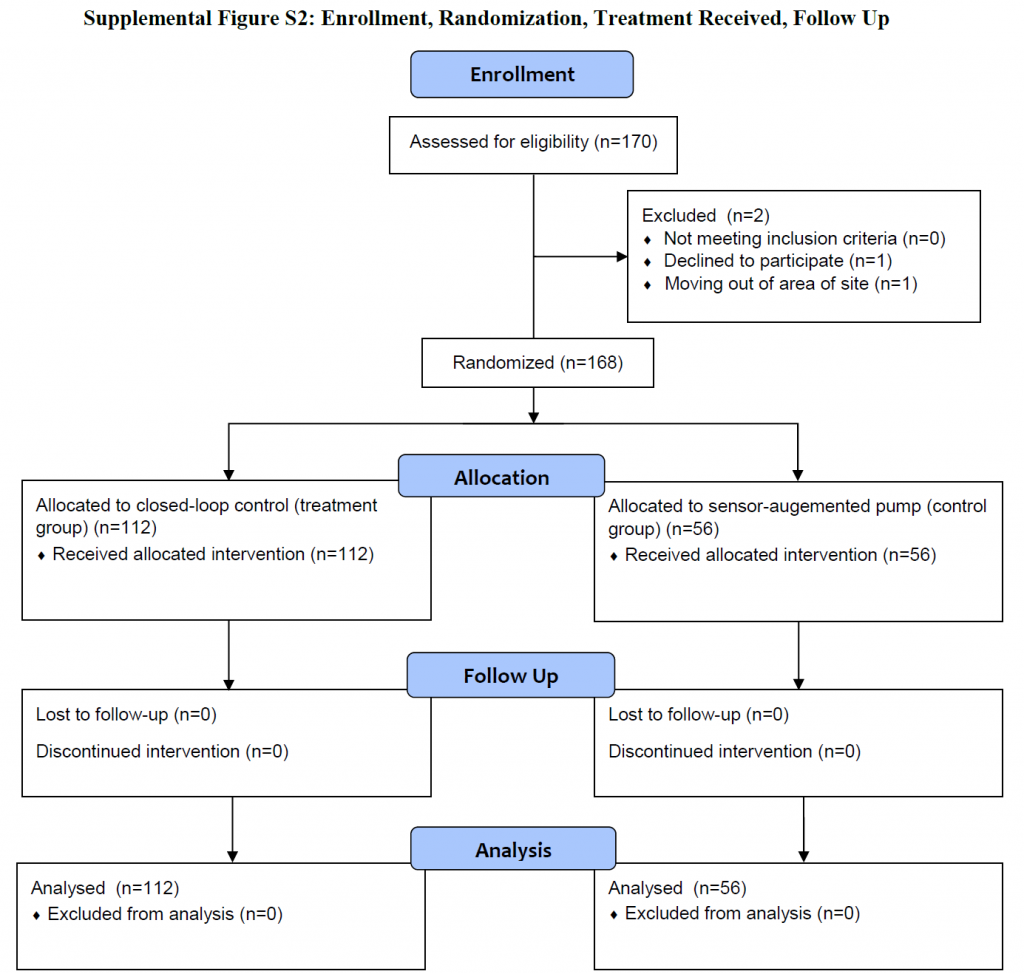
Were all of the patients who entered the trial properly accounted for at its conclusion?
In total 170 patients enrolled and were assessed for eligibility. 2 patients were excluded before the randomization process. The remaining 168 patients were subsequently randomized and all of them finished the trial.
Protocol deviation
During the trial was found a software error of the closed-loop software, which have led to temporary suspension. This error led to incorrect discontinuation of insulin delivery or to an incorrect bolus being given. It affected 33 patients and they used during this period open-loop system instead. The analyses included all data recorded during this period.
Outcomes
The primary outcome was the percentage of time that the glucose level was in the target range of 3.9 to 10.0 mmol/l (70 to 180 mg/dcl).
The main secondary outcomes were:
- the percentage of time that the glucose level was > 10 mmol/l (180 mg/dcl)
- the mean glucose concentration
- the glycated haemoglobin level
- the percentage of time that the glucose level was < 3,9 mmol/l (70 mg/dcl)
- the percentage of time that the glucose level was < 3.0 mmol/l (54 mg/dcl)
Safety outcomes included the frequency of severe hypoglycaemia, diabetic ketoacidosis, and other serious adverse events.
Analysis
A total of 168 patients were randomized in 2:1 ratio (closed-loop : control). Outcomes were summarised as percentages of time and percentages points with 95% confidence interval.
Statistical analyses were performed on an intention-to-treat basis, and all patients were included in the primary analysis and all secondary analyses.
Results
A total of 168 patients were randomly assigned to either the closed-loop group (112 patients) or the control group (56 patients).
The patients’ ages ranged in age (14 to 71 years), the duration of diabetes (1 – 62 years), and the baseline glycated haemoglobin level (5.4 – 10.6%).
Overall, 100% of follow-up visits and 99.9% of telephone contacts were completed. There were 68 unscheduled visits in the closed-loop group (37 to obtain supplies related to the trial, 28 related to a trial device issue, and 3 for other reasons) and 13 in the control group.
How large was the treatment effect?
The mean percentage of time with glucose levels within the target range increased from 61±17% at baseline to 71±12% during the 6 months in the closed-loop group and remained unchanged at 59±14% in the control group. The treatment effect was evident in the first month and was consistent over the 6 months.
How precise was the estimate of the treatment effect?
All the result (of primary as well as secondary outcomes) had confidence interval 95%.
Primary outcomes
The mean percentage of time with glucose levels within the target range of 3,9-10 mmol/l:
- increased from 61±17% at baseline to 71±12% during the 6 months in the closed-loop group
- remained unchanged at 59±14% in the control group
This meant a difference of 2.6 more hours per day spent in the target range in the closed-loop group. The treatment effect was consistent over the 6 months.
Secondary outcomes
All five main secondary outcomes met the prespecified criterion for significance in favour of the closed-loop system. Similar results favouring the closed-loop system were seen for the other secondary outcomes as well. Exact numbers can be found in original study.
There was no significant difference between closed-loop and control group in the daily insulin amount or weight change; the closed-loop group performed more glucose measurements.
There were 137 reported device problems, most commonly due to a connectivity problem.
Adverse events
A total of 17 adverse events were reported among 16 patients in the closed-loop group, and 2 adverse events were reported among 2 patients in the control group.
In the closed-loop group it was 1 case of diabetic ketoacidosis due to infusion set failure, 13 hyperglycemias or ketosis events and 3 other events (concussion, otitis, cardiac bypass surgery).
In control group it was 2 events of hyperglycaemia or ketosis.
Severe hypoglycaemia did not occur in either group.
The events of hyperglycaemia didn’t meet criteria for diabetic ketoacidosis.
Blood ketone levels >1.0 mmol/l were recorded in 11 patients in the closed-loop and in 8 patients in the control group.
Discussion
This trial shows that the closed-loop system led to a greater percentage of time that the glucose level was in a target range, less hyperglycaemia and hypoglycaemia, and better glycated haemoglobin levels than a sensor-augmented pump.
However, more adverse events were reported in the closed-loop group than in the control group, primarily as a result of hyperglycaemia with ketosis from pump infusion set failure. Therefore further improving of the software is necessary.
Were all clinically important outcomes considered?
Yes.
Most of the patients enrolling in the study were using a continuous glucose monitor and/or an insulin pump. This may reflect an interest in and willingness to use a closed-loop system among patients who were already using devices as part of diabetes management.
Are the benefits worth the harms and costs?
The benefits clearly outweight the risk. The percentage of time that blood glucose was in the target range was significantly higher in the closed-loop group than in the control group. This outcome reflected less time with glucose levels >10mmol/l or <3,9mmol/l. However, the proportion of patients with adverse events (total of 17 events in 16 patients) was higher in the closed-loop group.There were also more unscheduled contact visits in the closed-loop group. Most of these were a result of software failure.
The objective of the study was clearly stated, the study used valid methods to address the question the inclusion and exclusion criteria were stated. Randomization process was properly conducted. Patient characteristics, interventions, and outcomes were described. P values and CI were provided in some cases and in other cases mentioned as significant or not significant. The study was easy to analyse, the data is consistent and reliable. The results of the study are applicable to local population.
However, the error of the closed-loop software that lasted in some patients for up to 4 weeks might had influenced the results, as well as the fact, that one patient was using acarbose, a medication meeting exclusion criteria.
Can the results be applied to the local population, or in your context?
The trial population included both insulin-pump users and injection insulin users across a wide age range (14 to 71 years) and baseline range of glycated hemoglobin levels (5.4 to 10.6%), with consistent results favouring the closed-loop system.
Few other randomized trials have assessed a closed-loop system for 3 or more months. Anderson et.al stated in their study a 4.8-percentage-point higher percentage of time that blood glucose was within the range. Other trials in which closed-loop systems used a different algorithm (as in studies by Tauschmann et.al or Benhamoun et.al), state very similar results, favouring the closed loop system.
Several other studies regarding the closed-loop system are currently in progress, one of which is a study being conducted by McGill University in Montreal. Its aim is to develop a fully-automated, triple-hormone, closed-loop system that delivers insulin, pramlintide, and glucagon to regulate glucose levels without carbohydrate counting. Another one, sponsored by Manchester University NHS Foundation Trust, has the main objective to determine whether automated closed-loop system using faster-acting insulin aspart will improve glucose levels compared to insulin aspart.
The closed-loop systems offer the promise of improved glycemic control as long as system malfunctions are minimized. However, these current systems still require patients to dose premeal bolus insulin manually.
Author and attribution
Lucia Veronika Sepešiová is final year undergraduate medical student at Masaryk University, Czech Republic. No conflicts of interest have been disclosed.
11 questions from RCT checklist by Critical Appraisal Skills Programme have been used under non-commercial CC 3.0 license.
References
Brown SA et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019 Oct 31; 381:1707. [online] Available at: https://doi.org/10.1056/NEJMoa1907863 [Accessed 1.2.2020]
Supplement to: Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closedloop control in type 1 diabetes. N Engl J Med 2019; 381:1707-17. DOI: 10.1056/NEJMoa1907863
Nice.org.uk (2016): Diabetes (type 1 and type 2) in children and young people: diagnosis and management. [online] Available at: https://www.nice.org.uk/guidance/ng18/chapter/1-Recommendations#type-1-diabetes [Accessed 1.2.2020]
MayoClinic.org: Diabetes. [online] Available at: https://www.mayoclinic.org/diseases-conditions/diabetes/symptoms-causes/syc-20371444 [Accessed 1.2.2020]
Alvin C. Powers; Kevin D. Niswender; Michael R. Rickels: Chapter 397: Diabetes Mellitus: Management and Therapies, Harrison’s principles of internal medicine. [online] Available at: https://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192288412 [Accessed 1.2.2020]
Allan S. Brett, MD reviewing Brown SA et al. N Engl J Med 2019 Oct 3: Another “Closed-Loop” Insulin Delivery System. [online] Available at: https://www.jwatch.org/na50168/2019/11/12/another-closed-loop-insulin-delivery-system [Accessed 1.2.2020]
American Diabetes Association: Diabetes Care (2015 Jan); 38. (Supplement 1): S8-S16 [online] Available at: https://doi.org/10.2337/dc15-S005 [Accessed 1.2.2020]
American Diabetes Association. Glycemic targets: Standards of Medical Care in Diabetes — 2019. Diabetes Care 2019;42:Suppl 1:S61-S70.
Anderson SM. The International Diabetes Closed Loop Trial. Presented at the 18th Annual Diabetes Technology Meeting, Bethseda, MD, November 8–10, 2018.
CinicalTrials.gov: Identifier NCT03800875, Triple-hormone (Insulin-pramlintide-glucagon) Closed-loop Strategy to Regulate Glucose Levels Without Carbohydrate Counting (Triple); [online] Available at: https://clinicaltrials.gov/ct2/show/NCT03800875?term=closed+loop&recrs=abdf&type=Intr&cond=Diabetes+Mellitus%2C+Type+1&draw=2&rank=4 [Accessed 4.2.2020]
CinicalTrials.gov: Identifier NCT03579615, Comparison of Day and Night Closed-loop With Faster-acting Insulin Aspart With Insulin Aspart (AP-MFT-01); [online] Available at: https://clinicaltrials.gov/ct2/show/NCT03579615?term=closed+loop&cond=Diabetes+Mellitus&draw=3&rank=20 [Accessed 4.2.2020]