Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results
Pre-print
October 15, 2020
WHO Solidarity trial consortium, Hongchao Pan, Richard Peto, Quarraisha Abdool Karim, Marissa Alejandria, Ana Maria Henao-Restrepo, César Hernández García, Marie-Paule Kieny, Reza Malekzadeh, Srinivas Murthy, Marie-Pierre Preziosi, Srinath Reddy, Mirta Roses Periago, Vasee Sathiyamoorthy, John-Arne Røttingen, Soumya Swaminathan
DOI: doi.org/10.1101/2020.10.15.20209817
ClinicalTrials.gov: NCT04315948
Introduction
This large, adaptive, multicentre, open-label clinical trial has investigated re-purposed and potentially effective antivirals in treatment of COVID-19: Remdesivir, Lopinavir-Ritonavir combination, Hydroxychloroquine and Interferon β1a. This WHO-sponsored, locally-run and funded trial is still ongoing as of this writing, however, these interim preliminary results on mortality have been published before the trial’s completion. Since the trial is adaptive, Hydroxychloroquine and Interferon have been excluded as they showed unpromising results. Monoclonal antibodies, which will be added to the trial later, have not yet been reported on.
Methods
Study design
This trial was adaptive, randomised, multicentre, and open-label.
The trial was WHO- and government-sponsored in each participating country with most of the costs covered by the countries themselves. Respective drugs were donated by pharmacological companies manufacturing those drugs.
The main objective of the trial was to determine the effect of studied drugs on in-hospital mortality in patients with COVID-19.
Did the trial address a clearly focused issue?
Yes.
The trial’s simple design and straightforwardly-defined outcomes have accurately zoomed-in on the questions of crude efficacy of studied drugs.
Eligibility
Inclusion criteria were the following:
- Age ≥18 years
- Patients hospitalized with a diagnosis of COVID-19
- Patients who have not received any study drug
- Patients without anticipated transfer elsewhere within 72 hours
- Patients without contra-indication to any study drug
Exclusion criteria
- Patients without clear consent to follow-up
Deviations from eligibility
- None have been reported.
Study protocol
Was the assignment of patients to treatments randomised?
Yes.
In 405 hospitals in 30 countries around the world, 11 266 adults were enrolled. They were divided into 5 groups (4 drugs or local standard of care group), based on local drug availability. This randomisation process was online-based and same for all the hospitals. The controls were those patients who at respective hospitals were randomly allocated to Standard of Care (SoC) only, instead of the locally-administered drug. If more than 1 drug was available at the hospital, the control was for each of those drugs. That is why the control groups partially overlap. The comparisons in the analyses were evenly randomized afterwards, so that the patient characteristics were well balanced.
Were the groups similar at the start of the trial?
Yes. Reported patient characteristics were similar in all groups.
Were patients, health workers, and study personnel ‘blind’ to treatment?
No. The study states that ‘the mortality findings cannot have been appreciably biased by the open-label design without placebos’.
Aside from the experimental intervention, were the groups treated equally?
Yes. All hospitals used the same drug protocols and dosages. However, SoC differed across countries. This bias has been minimised by the above-mentioned unstratified randomisation. Even in absolute numbers, for example in use of corticosteroids and other non-study treatments, the differences were small.
Were all of the patients who entered the trial properly accounted for at its conclusion?Yes.

2750 patients were allocated Remdesivir, 954 Hydroxychloroquine, 1411 only Lopinavir-ritonavir, 2063 Interferon, and 4088 no study drug.
Protocol deviation
No deviation or change has been reported.
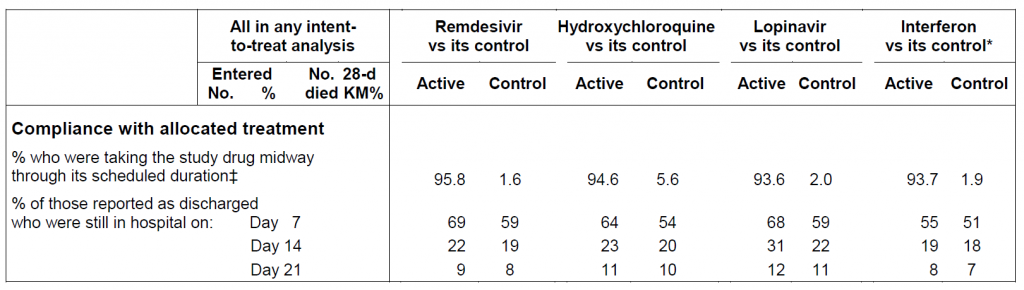
Compliance itself was reported to be 94-96% in the middle of treatment, with 2-6% crossover. Compliance was calculated only in those who died or were discharged alive.
Outcomes
The primary objective was to find out the in-hospital mortality in all, moderate, and severe COVID-19.
The secondary outcomes were
- initiation of ventilation
- hospitalization duration
Analysis
The intention-to-treat analyses were done. Separate analyses were performed for all, moderately, and severely affected patients (defined as ventilated at the start of treatment).
The risk of death used Kaplan-Meier plot. Death rate ratios (RRs) and p-values are from log-rank analyses divided into six categories of age and ventilation at entry.
For overall mortality, 95% Confidence Intervals (Cis) are available. 99% CI for subgroup analyses.
Results
Primary outcome
Not one of the re-purposed antivirals has had any effect on mortality, either overall (each p>0.10) or in any subgroup (age, ventilation at entry, or other entry characteristics, geographic region, corticosteroid use).
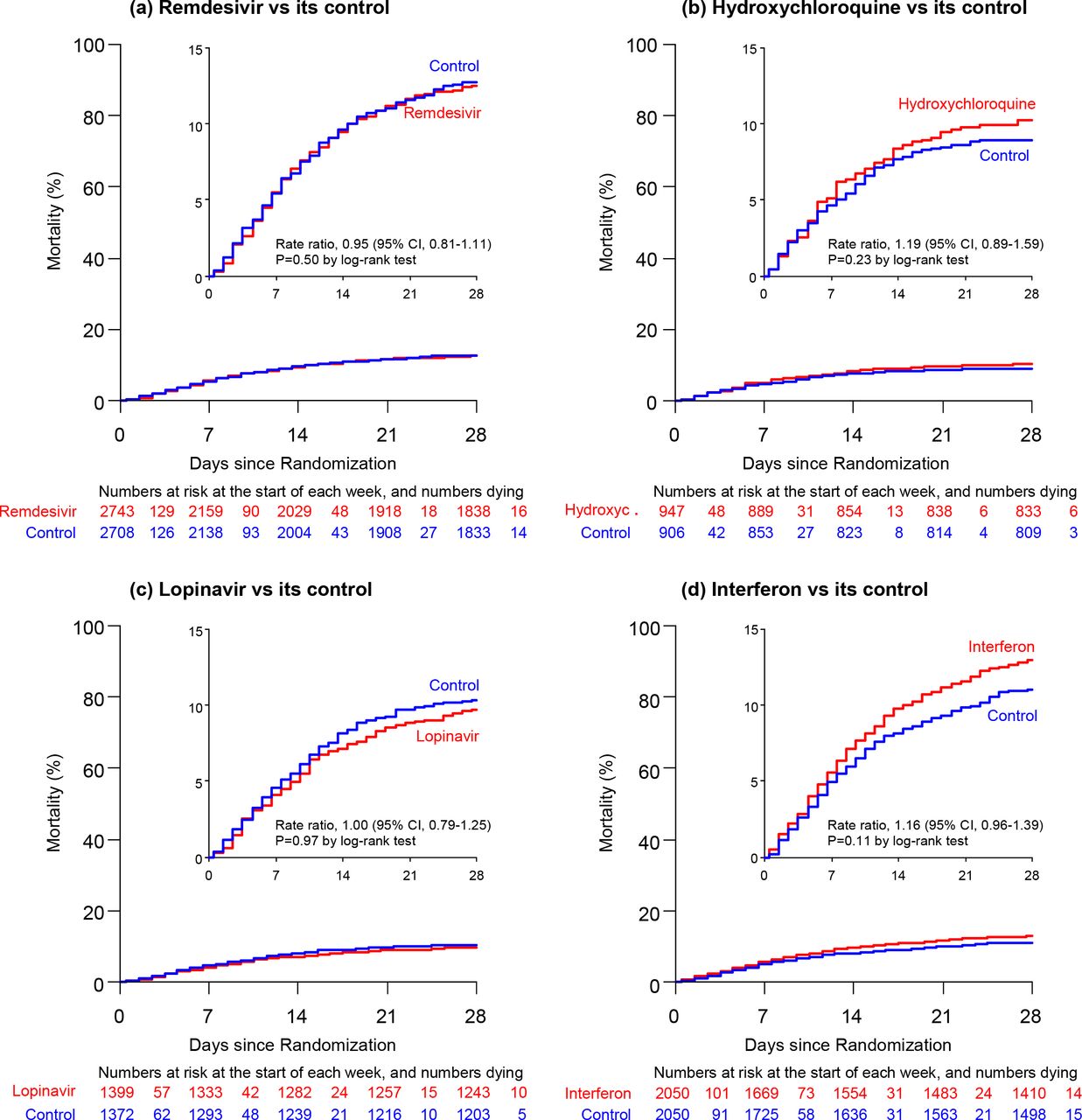
Death rate ratios (with 95% CIs, and drug vs control numbers of deaths thus far reported) were:
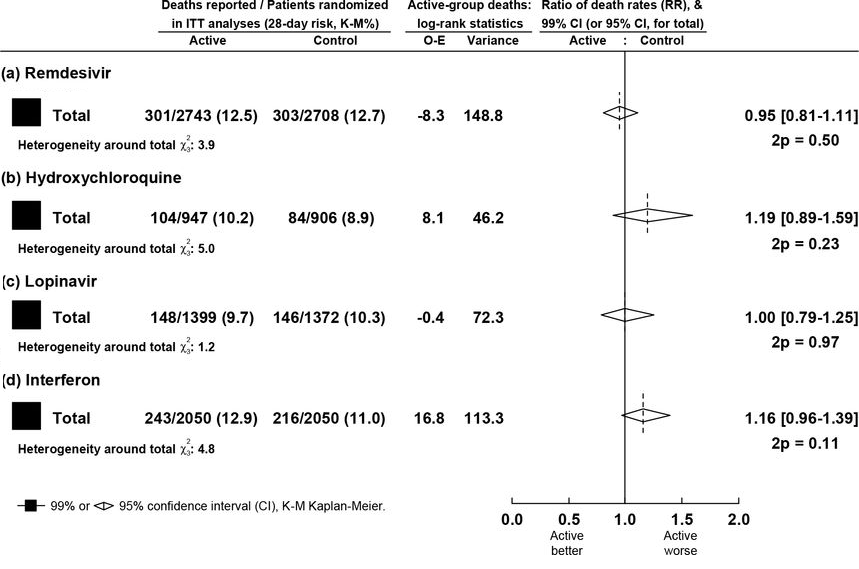
Neither other analyses, for specific groups (without corticosteroid use, or all 4 drugs together) have found any effect on mortality.
11.8% were estimated to die at day 28 using Kaplan-Meier plot. This risk depended on several factors, particularly:
- age (20% if ≥70 years, 6% if <50 years)
- ventilation (39% if ventilated, otherwise 10%).
Secondary outcomes
Ventilation or ventilation initiation were found not to be reduced by neither of the study drugs. Hospitalization duration was unaffected by the drugs.
Discussion
Since the start of the pandemic, many potentially effective drugs for COVID-19 have been identified. Amongst the most promising drugs, in terms of mortality reduction, are those studied in this WHO Solidarity trial. It is, therefore, disappointing that no observable reduction in mortality has been found even despite a large international cohort of patients, simple study protocol, high compliance, and robust reporting.
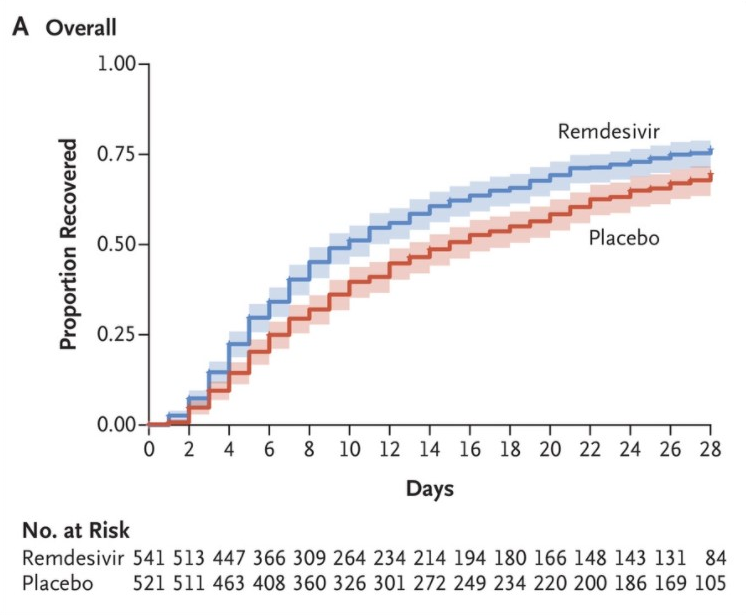
Remdesivir had been investigated in large controlled trials before. In the adaptive, placebo-controlled, double-blinded NIAID ACTT-1 trial, Remdesivir was reported to have a positive effect on the median time to recovery: for Remdesivir group (n=541) it was 10 days (95% CI 9-11) vs. 15 days (95% CI 13-18) in the placebo group (n=521). Similarly, mortality was less in remdesivir group (11.4%) than in the placebo group (15.2%) at day 29 (HR 0.73, 95% CI 0.52-1.03). In the severe disease stratum (957 patients) the median time to recovery was 11 days, as compared with 18 days (rate ratio for recovery, 1.31; 95% CI, 1.12 to 1.52).
Next, the randomized, double-blind, placebo-controlled, multicentre trial from Hubei, China, enrolled into the intention-to-treat population only 237 participants (41% women, median age 65). There, remdesivir resulted in no significant difference in time to clinical improvement versus placebo (hazard ratio 1.23, 95% CI 0.87-1.75). But, after stopping early these results have been deemed unreliable as the trial was found to be underpowered.
Spinner et al, have conducted a randomised, open-label phase 3 trial (called the SIMPLE study), which has shown no clear clinical benefit. The Kaplan-Meier estimates of all-cause mortality at day 28 were 1% (95% CI, 0.0%-2.6%) for the 5-day remdesivir group (log-rank P = .43 vs standard care), 2% (95% CI, 0.0%-3.6%) for the 10-day remdesivir group (log-rank P = .72 vs standard care), and 2% (95% CI, 0.1%-4.1%) for the standard care group.
As regards clinical status however, the SIMPLE study has shown that those moderately-affected patients who had a 5-day course of remdesivir had a statistically significantly better clinical status compared with those randomized to standard care at 11 days after initiation of treatment.
Combining data from these four trials yielded Remdesivir vs control death rate ratio (RR) of 0.91 (95% CI 0.79-1.05). As mentioned by the authors in the discussion, these four clinical trials provide most of the evidence for Remdesivir effect on mortality. They combined the results of all four, but only ACTT-1 and Solidarity trials can be taken seriously as the third was underpowered and fourth was imprecise in terms of mortality.
These two had larger cohorts of studied patients and yet, the definitive answer has not been reached. Clinical status of those might have improved compared to placebo in ACTT-1 trial, but that remarkable median time to recovery difference was undercut by the statistical insignificance of overall mortality. On the contrary, the Solidarity’s larger studied population, and thus, more statistical weight, should have cleared the muddy Remdesivir waters. But the narrower CI of overall mortality unfortunately includes 1.0 as well.
Can the results be applied to the local population, or in your context?
Yes. A thorough randomization, a larger cohort, and a multicentre setting have ensured accurate reproducibility. According to this study’s results, the amount of dying patients with COVID-19 would not be affected by Remdesivir use.
Were all clinically important outcomes considered?
No. Secondary outcomes could have copied ACTT-1 trial’s design more. It would have certainly been more interesting to compare these outcomes directly, since the SOLIDARITY’s larger population could have proven or disproven the results in terms of time to recovery. Even though the authors suggest that effect on the ventilation initiation or time to discharge in this study should weigh in more in the argument, the ACTT-1’s results measured slightly different outcomes and have proven some benefit.
Are the benefits worth the harms and costs?
Electronic medicines compendium mentions elevated transaminases as the most common adverse effect in healthy patients (Very common (≥1/10). Common adverse effects (≥1/100 to <1/10) are nausea, headache, and rash. Rare (≥1/10,000 to <1/1,000) events involve mainly hypersensitivities.
A five day-course has been calculated at about £1,900. It is not as great a cost-benefit ratio as the cheap corticosteroids, namely dexamethasone, which are also used in treatment of COVID-19, but if it somewhat shortens the in-hospital stay of some of the patients with COVID-19, it might well be worth the cost, even though it most probably does not save many lives.
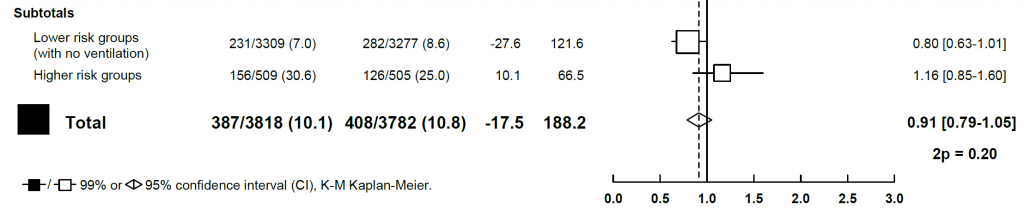
How big an effect does Remdesivir have on mortality? Overall, the mortality might be positively affected, mainly in those who are in lower-risk groups. The CI of the metanalyses crosses the value 1.0 by only 0.1. On the contrary, those already ventilated have little probability of benefitting from Remdesivir use.
When it comes to other 3 reported drugs, Interferon-β1 has been the second most promising after Remdesivir. Unfortunately, the SOLIDARITY’s data cannot be compared with another large-cohort mortality study as none have been conducted. Saddening is, even subgroup analyses have not shown mortality reductions. Not even in the lower-risk group as CI of ratio of death rates indifferently crosses 1.0 (0.11 [0.84-1.45]).
The other two drugs, Lopinavir and Hydroxychloroquine have been excluded by the investigators. No benefit in terms of mortality reduction has been found.
To conclude, the SOLIDARITY’s interim data have thus far disproven any claim that any of these four drugs has any major effect on mortality. Although we will have to wait for the final report, it is highly improbable that the finalised data will in any way contradict already published findings.
Author and attribution
Tomáš Poliačik is a Junior Clinical Fellow at St Helens and Knowsley Teaching Hospitals NHS Trust. No conflicts of interest have been disclosed.
11 questions from RCT checklist by Critical Appraisal Skills Programme have been used under non-commercial CC 3.0 license.
References
Beigel, J.H., Tomashek, K.M., Dodd, L.E., Mehta, A.K., Zingman, B.S., Kalil, A.C., Hohmann, E., Chu, H.Y., Luetkemeyer, A., Kline, S., Lopez de Castilla, D., Finberg, R.W., Dierberg, K., Tapson, V., Hsieh, L., Patterson, T.F., Paredes, R., Sweeney, D.A., Short, W.R., Touloumi, G., Lye, D.C., Ohmagari, N., Oh, M., Ruiz-Palacios, G.M., Benfield, T., Fätkenheuer, G., Kortepeter, M.G., Atmar, R.L., Creech, C.B., Lundgren, J., Babiker, A.G., Pett, S., Neaton, J.D., Burgess, T.H., Bonnett, T., Green, M., Makowski, M., Osinusi, A., Nayak, S. and Lane, H.C. (2020). Remdesivir for the Treatment of Covid-19 — Preliminary Report. New England Journal of Medicine.
Editor, J.D., US Business (2020). Covid drug remdesivir to cost £1,900 for five days. www.thetimes.co.uk. [online] 30 Jun. Available at: https://www.thetimes.co.uk/article/covid-drug-to-cost-1-900-for-five-days-9zn5vbr0c [Accessed 30 Oct. 2020].
Pan, H., Peto, R., Abdool Karim, Q., Alejandria, M., Henao Restrepo, A.M., Hernandez Garcia, C., Kieny, M.P., Malekzadeh, R., Murthy, S., Preziosi, M.-P., Reddy, S., Roses, M., Sathiyamoorthy, V., Rottingen, J.-A. and Swaminathan, S. (2020). Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results.
Spinner, C.D., Gottlieb, R.L., Criner, G.J., Arribas López, J.R., Cattelan, A.M., Soriano Viladomiu, A., Ogbuagu, O., Malhotra, P., Mullane, K.M., Castagna, A., Chai, L.Y.A., Roestenberg, M., Tsang, O.T.Y., Bernasconi, E., Le Turnier, P., Chang, S.-C., SenGupta, D., Hyland, R.H., Osinusi, A.O., Cao, H., Blair, C., Wang, H., Gaggar, A., Brainard, D.M., McPhail, M.J., Bhagani, S., Ahn, M.Y., Sanyal, A.J., Huhn, G. and Marty, F.M. (2020). Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19. JAMA.
Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., Fu, S., Gao, L., Cheng, Z., Lu, Q., Hu, Y., Luo, G., Wang, K., Lu, Y., Li, H., Wang, S., Ruan, S., Yang, C., Mei, C., Wang, Y., Ding, D., Wu, F., Tang, X., Ye, X., Ye, Y., Liu, B., Yang, J., Yin, W., Wang, A., Fan, G., Zhou, F., Liu, Z., Gu, X., Xu, J., Shang, L., Zhang, Y., Cao, L., Guo, T., Wan, Y., Qin, H., Jiang, Y., Jaki, T., Hayden, F.G., Horby, P.W., Cao, B. and Wang, C. (2020). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet, [online] 0(0). Available at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/fulltext.
www.medicines.org.uk. (2020). Veklury 100 mg concentrate for solution for infusion – Summary of Product Characteristics (SmPC) – (emc). [online] Available at: https://www.medicines.org.uk/emc/product/11596/smpc [Accessed 30 Oct. 2020].