Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19
NEJMoa2100433
February 25, 2021
REMAP-CAP Investigators; Anthony C Gordon, Paul R Mouncey, Farah Al-Beidh, Kathryn M Rowan, Alistair D Nichol, Yaseen M Arabi, Djillali Annane, Abi Beane, Wilma van Bentum-Puijk, Lindsay R Berry, Zahra Bhimani, Marc J M Bonten, Charlotte A Bradbury, Frank M Brunkhorst, Adrian Buzgau, Allen C Cheng, Michelle A Detry, Eamon J Duffy, Lise J Estcourt, Mark Fitzgerald, Herman Goossens, Rashan Haniffa, Alisa M Higgins, Thomas E Hills, Christopher M Horvat, Francois Lamontagne, Patrick R Lawler, Helen L Leavis, Kelsey M Linstrum, Edward Litton, Elizabeth Lorenzi, John C Marshall, Florian B Mayr, Daniel F McAuley, Anna McGlothlin, Shay P McGuinness, Bryan J McVerry, Stephanie K Montgomery, Susan C Morpeth, Srinivas Murthy, Katrina Orr, Rachael L Parke, Jane C Parker, Asad E Patanwala, Ville Pettilä, Emma Rademaker, Marlene S Santos, Christina T Saunders, Christopher W Seymour, Manu Shankar-Hari, Wendy I Sligl, Alexis F Turgeon 1, Anne M Turner, Frank L van de Veerdonk, Ryan Zarychanski, Cameron Green, Roger J Lewis, Derek C Angus, Colin J McArthur, Scott Berry, Steve A Webb, Lennie P G Derde
PMID: 33631065
DOI: 10.1056/NEJMoa2100433
ClinicalTrials.gov: NCT02735707
Introduction
After more than a year of pandemic of SARS-CoV-2 virus there is still no curative treatment available. Many drugs have been investigated and only few of them have solid evidence. Milestone in therapy has been a RECOVERY trial with dexamethasone use reducing mortality in severe COVID-19 with oxygen requirement. Its antiinflammatory effects are probably the reason.
Cytokine storm is now being increasingly more recognized as a potential cause of deterioration and multiorgan failure in critically ill, including those with COVID-19. One of their mediators – interleukin 6 (IL-6) – is a target for monoclonal antibodies investigated in this trial – tocilizumab and sirulimab.
Methods
Design
Trial was part of an adaptive platform trial for investigation of treatment strategies in patients with severe pneumonia not just in pandemic – Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP).
Randomized (with preferential assignment to the most favorable interventions)
Open label
No placebo controlled
Its COVID-19 Immuno Modulation Therapy domain investigated:
- Control (no immune modulation, no placebo)
- interferon β1a
- anakinra
- tocilizumab
- sarilumab
Roche Products and Sanofi provided tocilizumab and sarilumab in the UK but had no other role in the trial.
Eligibility
Eligible patients had to have following criteria:
- Critically ill
- >18 years old
- COVID-19 suspected or confirmed
- admitted to ICU
- receiving respiratory or cardiovascular support
Patients were excluded if:
- death imminent
- not for full support
- previously participated in REMAP-CAP trial in 90 days
Protocol
Patients at each site were randomized to a control group and at least 1 intervention.
Central computer assigned patients to groups of tocilizumab, sarilumab or control (depending on availability at each site).
Tocilizumab – 8 mg/kg (actual body weight) IV, maximum 800 mg. Could have been repeated once in 12-24 hours if no response.
Sarilumab – 400 mg IV once only.
Outcomes
Primary outcome
Number of days free of respiratory and cardiovascular organ support up to day 21.
Secondary outcome
- Hospital mortality or survival
- 90-day survival
- Respiratory support-free days
- Cardiovascular support-free days
- Time to ICU discharge
- Time to hospital discharge
- WHO ordinal scale (0-8)
- Progression to invasive mechanical ventilation, ECMO or death
Statistical analysis
The trial used Bayesian statistics. Primary analysis was done by statistical analysis committee. Patients with severe disease were enrolled but the primary model included also patients not only in the immune modulation domain (that means patients in REMAP-CAP trial in other treatment groups outside of those reported here).
Principles of this statistical analysis are quite complex – to understand fully I suggest you read the full text.
It is not clear if people analyzing data were blinded.
Did the study address a clearly focused research question?
Yes
Was the assignment of participants to interventions randomised?
Yes
Were all participants who entered the study accounted for at its conclusion?
No. Some patients withdrew consent or outcome was not available.
Were the participants ‘blind’ to intervention they were given?
No. This was an open-label study.
Were the investigators ‘blind’ to the intervention they were giving to participants?
No.
Were the people assessing/analysing outcome/s ‘blinded’?
Not sure.
Results
| Baseline characteristics | Tocilizumab(N=353) | Sarilumab(N=48) | Control(N=402) |
| Age — yr | 61.5±12.5 | 63.4±13.4 | 61.1±12.8 |
| Body-mass index median (IQR) | 30.5 (26.9–34.9) | 29.2 (26.0–33.8) | 30.9 (27.1–34.9) |
| Median time to enrollment (IQR) | |||
| From hospital admission — days | 1.2 (0.8–2.8) | 1.4 (0.9–2.8) | 1.2 (0.8–2.8) |
| From ICU admission — hr | 13.1 (6.6–19.0) | 16.0 (11.4–20.8) | 14.0 (6.8–19.5) |
| Acute respiratory support — no./total no. (%) | |||
| None or supplemental oxygen only | 1/353 (<1) | 0/48 | 2/402 (<1) |
| High-flow nasal cannulae | 101/353 (29) | 17/48 (35) | 110/402 (27) |
| Noninvasive ventilation only | 147/353 (42) | 23/48 (48) | 169/402 (42) |
| Invasive mechanical ventilation | 104/353 (29) | 8/48 (17) | 121/402 (30) |
| Vasopressor support — no./total no. (%) | 63/353 (18) | 4/48 (8) | 79/402 (20) |
| PaO2:FiO2 Median (IQR) | 115 (89–162) | 126 (99–157) | 118 (89–169) |
| C-reactive protein median (IQR) – μg/ml | 150 (85–221) | 136 (105–204) | 130 (71–208) |
Were the study groups similar at the start of the randomised controlled trial?
Yes, as can be seen in baseline characteristics, although proportion of noninvasive or invasive ventilation patients differed due to sample size.
Apart from the experimental intervention, did each study group receive the same level of care (that is, were they treated equally)?
Institutional practices may have differed in the control group as standard of care was not defined in the protocol. Also the tocilizumab group may have had 2 doses or just one – this was at the discretion of the attending physician.
Primary outcome
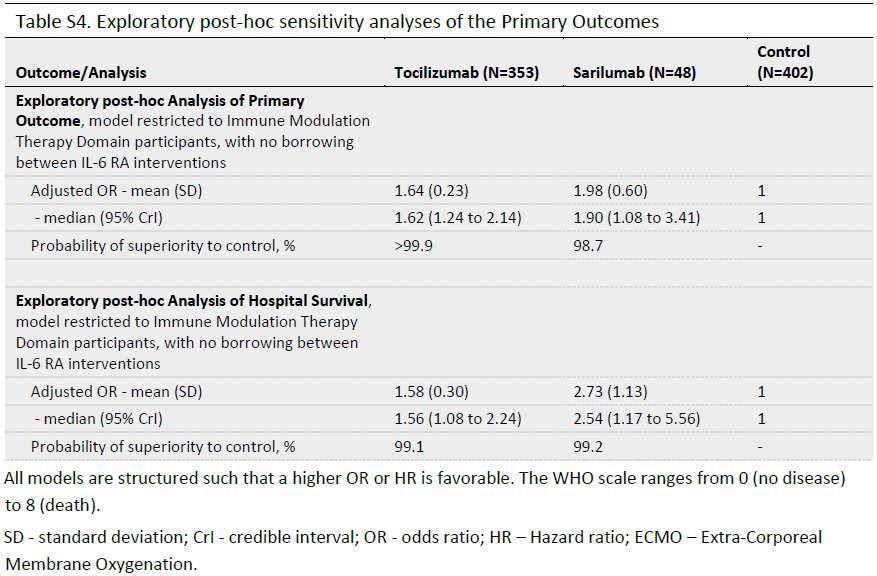
Secondary outcomes
Were the effects of intervention reported comprehensively?
I don’t know. Very little data points have been reported and those mainly consist of probability of superiority based on modelling mentioned above.
Was the precision of the estimate of the intervention or treatment effect reported?
Interquartile range has been quite large for primary outcome (organ support-free days).
Discussion
Patients with critical COVID-19 appear to have markedly elevated inflammatory markers, including C-reactive protein, D-dimer, ferritin and cytokines like interleukin 6. Florid inflammatory reaction resembles a cytokine storm, although in COVID-19 cytokine levels are much lower.
However, it seems that in patients with organ failure, mainly with respiratory failure requiring supplemental oxygen, use of glucocorticosteroid dexamethasone reduces 28-day mortality. It would only make sense that use of an agent targeted against pro-inflammatory cytokine IL-6 would reduce the inflammation and, hopefully, improve outcomes.
Currently there are numerous randomized controlled trials available for tocilizumab (CORIMUNO-TOCI, EMPACTA, RCT-TCZ-COVID-19, BACC Bay Tocilizumab Trial, TOCIBRAS, RECOVERY, REMAP-CAP) and only few of them (REMAP-CAP and RECOVERY) demonstrated benefit in mortality.
Difficulty with COVID-19 therapeutic trials is that standard of care often changes during the trial and therefore patient population and treatment they are receiving is not consistent. Trials also adapt and change as new evidence emerges. In REMAP-CAP trial majority of patients had been enrolled after dexamethasone was found to be beneficial in RECOVERY trial. Also in this trial, 33% patients were receiving remdesivir.
| Trial | Population | Control | % receiving steroid | Finding |
| RECOVERY | Severe COVID-19 | Standard of care | 88% | Reduced 28-day mortality, 29% vs. 33%, RR 0.86 95% CI 0.77-0.96) |
| COVACTA | Severe COVID-19 | Placebo | 42% | No 28-day mortality benefit |
| EMPACTA | Severe COVID-19 not mechanically ventilated | Placebo | 83% | No 28-day survival benefit |
| CORIMUNO-TOCI-1 | Moderate or severe COVID, not mechanically ventilated | Standard of care | 48% | Fewer (24%) needing NIV, MV or died, vs. 36% |
| RCT-TCZ-COVID-19 | Hospitalized COVID-19 | Standard of care | 4% | No difference at worsening at 14 days |
It also seems that the sicker the patients the greater the benefit of the tocilizumab. This may be due to an additive effect to dexamethasone. Current recommendations of 6 mg OD of dexamethasone may not be adequate for critically ill patients. More data is needed on use of high-dose corticosteroid in critically ill patients, for example when tocilizumab or other IL-6 receptor antagonists are not available.
Tocilizumab does not work as a monotherapy without corticosteroids and it may be that blockade of one inflammatory cytokine and one part of inflammatory pathways may not be enough.
Do the benefits of the experimental intervention outweigh the harms and costs?
Yes. Tocilizumab is a well known RA drug and is usually well tolerated. Apart from general side effects possible with any drugs, hypersensitivity reactions, leukopenia and neutropenia are of significance. With a price of £102.40 for 80 mg vial it’s quite costly drug (roughly £700 for 70 kg person). However, single dose is an advantage and the cost of even one day at ICU is more costly.
Can the results be applied to your local population/in your context?
Yes. Most of these results have been collected from the UK population where now tocilizumab is being used in severe COVID-19.
Would the experimental intervention provide greater value to the people in your care than any of the existing interventions?
No. Tocilizumab is now being used across the UK.
Current recommendations
As of today, when publishing this article, current recommendations on use of IL6 blockers are:
National Institutes of Health (NIH): Hospitalized patients on corticosteroids on HFNO or greater and admitted to ICU <24 hours ago or have raised inflammatory markers.
NHS England: Hospitalized patients with confirmed/suspected COVID-19 + receiving corticosteroids (or unless contraindicated) + CRP >75 mg/L + SpO2 <92% on room air or on oxygen.
Attribution
11 questions from RCT checklist by Critical Appraisal Skills Programme have been used under non-commercial CC 3.0 license.
References
“Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19”. (2021), New England Journal of Medicine, Vol. 0 No. 0, p. null.
Rosas, I.O., Bräu, N., Waters, M., Go, R.C., Hunter, B.D., Bhagani, S., Skiest, D., et al. (2021), “Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia”, New England Journal of Medicine, available at:https://doi.org/10.1056/NEJMoa2028700.
Salama, C., Han, J., Yau, L., Reiss, W.G., Kramer, B., Neidhart, J.D., Criner, G.J., et al. (2021), “Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia”, New England Journal of Medicine, Vol. 384 No. 1, pp. 20–30.
Pulmonary, S.M.F. is the creator of P. org H. is an associate professor of and Vermont, C.C.M. at the U. of. (2021), “PulmCrit – Six RCTs to answer one question: what is the role of tocilizumab in COVID-19?”, EMCrit Project, 12 January, available at: https://emcrit.org/pulmcrit/tocilizumab/ (accessed 8 April 2021).
“COVID-19: Clinical features – UpToDate”. (n.d.). , available at: https://www.uptodate.com/contents/covid-19-clinical-features?search=covid%2019%20tocilizumab&topicRef=127429&source=see_link (accessed 8 April 2021).
“COVID-19: Management in hospitalized adults – UpToDate”. (n.d.). , available at: https://www.uptodate.com/contents/covid-19-management-in-hospitalized-adults?search=covid%2019%20tocilizumab§ionRank=1&usage_type=default&anchor=H2290242517&source=machineLearning&selectedTitle=1~150&display_rank=1#H2290242517 (accessed 8 April 2021).
“3 The technologies | Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed | Guidance | NICE”. (n.d.). , available at: https://www.nice.org.uk/guidance/ta375/chapter/3-The-technologies#tocilizumab (accessed 8 April 2021).
“Coronavirus » Interim Clinical Commissioning Policy: Tocilizumab for hospitalised patients with COVID-19 pneumonia (adults)”. (n.d.). , available at: https://www.england.nhs.uk/coronavirus/publication/interim-clinical-commissioning-policy-tocilizumab-for-hospitalised-patients-patients-with-covid-19-pneumonia-adults/ (accessed 8 April 2021).